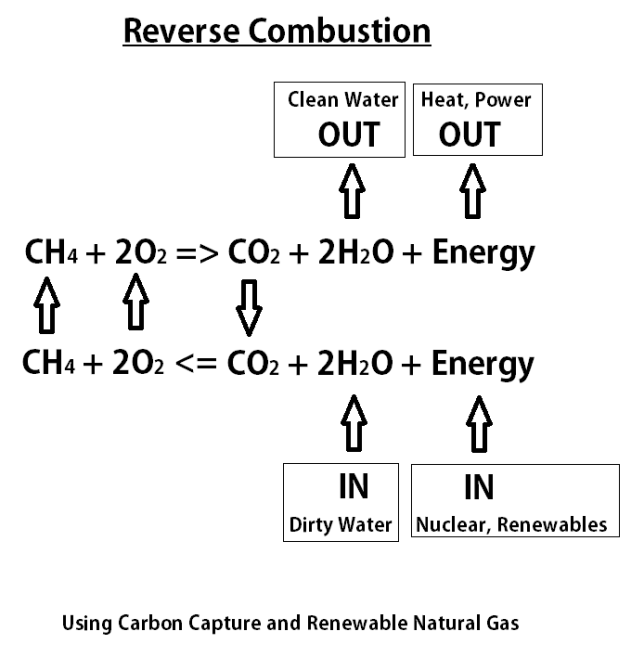
This equation describes the idea of reverse combustion with renewable methane, carbon capture and oxy-combustion. This is also known as chemical looping and is a form of using renewable natural gas as energy storage.
Combustion works by combining fuel (CH4) with oxygen to create heat (energy) plus carbon dioxide (CO2) and water (H2O). Typically the CO2 and H2O are just released to the atmosphere, but if we capture the CO2 to prevent it from going to the atmosphere as a greenhouse gas, then we can do reverse combustion and recycle it.
Reverse combustion requires an energy input such as nuclear power or renewables to perform electrolysis on the CO2 and water to create renewable methane and oxygen. The oxygen must be looped back and reused for oxy-combustion to enables the carbon capture.
The use of water in this equation is interesting because non-drinking water can be used for the electrolysis and clean water can be produced on the back end creating an opportunity to use power production as a means to produce clean water.
The combustion of 1 pound of methane results in the production of 3.71 gallons of water and 1 Bcf of methane produces over 11 million gallons of water.